free-ZBi
Understanding Zn self-discharge into free Zinc-MnO2 Batteries
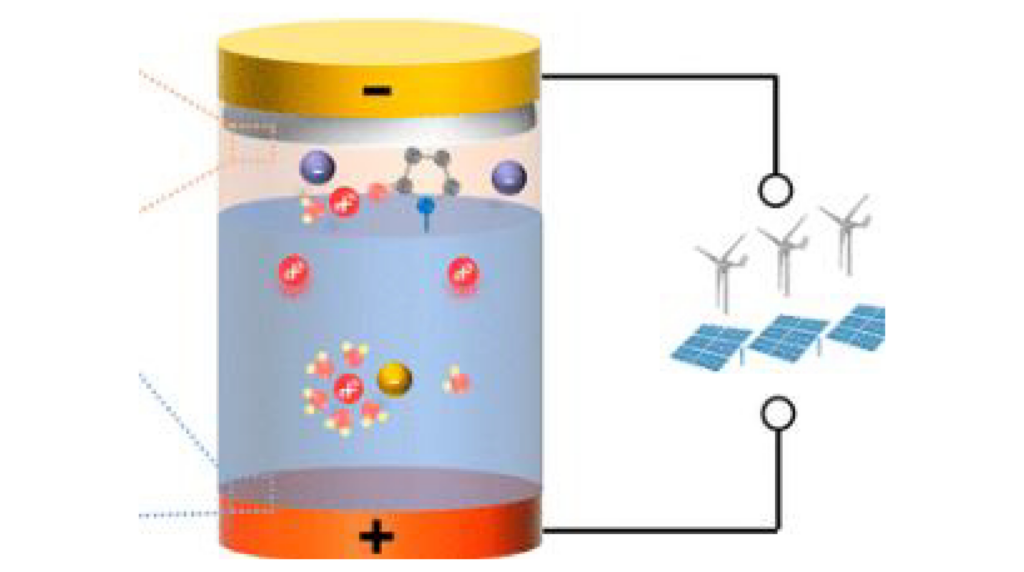
Given the intensifying environmental pollution and the energy crisis associated with the combustion of fossil fuels, there is an urgent need to establish a low-carbon society relying on electrochemical energy storage systems. Today, one of the challenges in developing batteries for energy storage is to design batteries with storage durations storage ranging from few hours to 12 hours at low cost. Recently, rechargeable zinc-ion batteries have garnered significant interest as promising candidates for this advanced energy storage. Their low equilibrium potential of Zn (-0.76 V vs SHE) and the use of aqueous electrolyte make them particularly attractive, offering advantages such as affordability, reduced flammability, high energy densities, and environmental friendliness. However, Zn-MnO2 batteries, the most prominent and promising Zn-ion battery technologies, exhibit significant self-discharge due to zinc dissolution and HER promotion. Interestingly, in mild acidic aqueous Zn-MnO2 batteries, the corrosion and self-discharge issues have been overlooked because these batteries are commonly packed with excess of Zn, compensating for corrosion issue, making the study of self-discharge challenging.
The free-ZBi project focuses on developing new electrode-free Zn-MnO2 batteries enabling a rational use of resources. It uses electrospun carbon nanofibers papers as current collectors for electrodes. However, this requires focusing on self-discharge problems and in particular offering alternative electrolytes in which these are minimized. Combining optical microscopy with electrochemistry will aid us to comprehend and diagnose self-discharge from such operando monitoring. Once the understanding of Zn corrosion is attained, the free-ZBi project will delve into designing biphasic electrolyte to enhance the battery’s durability. Interestingly, this concept allows the design of membrane-free batteries.