Characterization of Li+ Transport through the Organic-Inorganic Interface by using Electrochemical Impedance Spectroscopy
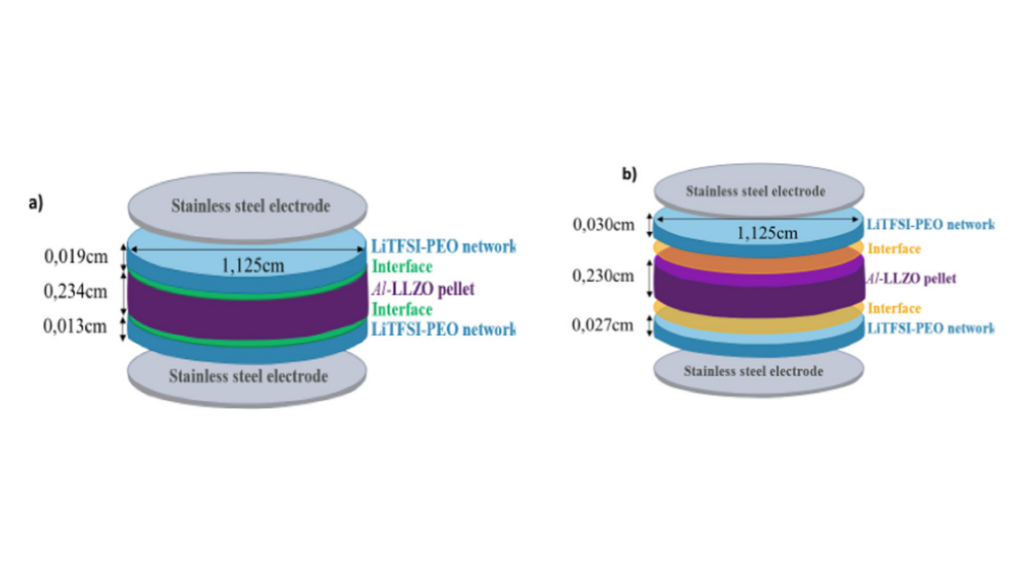
Understanding Li+ transport at polymer||inorganic interfaces is crucial for developing composite electrolytes in solid-state batteries. In our investigation, we employed impedance spectroscopy and established a multilayer methodology for assessing Li+ transport at this interface. The inorganic phase chosen was Li6.25Al0.25La3Zr2O12 (Al−LLZO), and the organic phase comprised a Poly(ethylene oxide) (PEO) network with dangling chains. Li+ incorporation in the polymer, as a free either salt or associated with anion grafting onto the PEO network, was explored. Additionally, the PEO network was either pressure-adhered to the inorganic surface (ex-situ configuration) or synthesized onto the Al−LLZO surfaces (in situ configuration) to investigate processing effects on Li+ transport. Using a Transmission Line Model for impedance data analysis, our study identified two key elements governing Li+ transport at the interface: Ri, representing resistance along the ionic pathway, and Rt and Ct, describing distributed resistance and capacitance within the interface. We observed that Ri is influenced by the polymerization process in the presence of Al−LLZO ceramic, while Rt remains constant regardless of the synthesis method. This suggests varying Li+ concentrations at the interphase in the in situ configuration, while interface/interphase heterogeneity remains consistent across configurations. The estimated activation energy indicates more energetically favorable direct Li+ transport in the in−situ configuration.