Chemical Decomposition of the TFSI Anion under Aqueous Basic Conditions
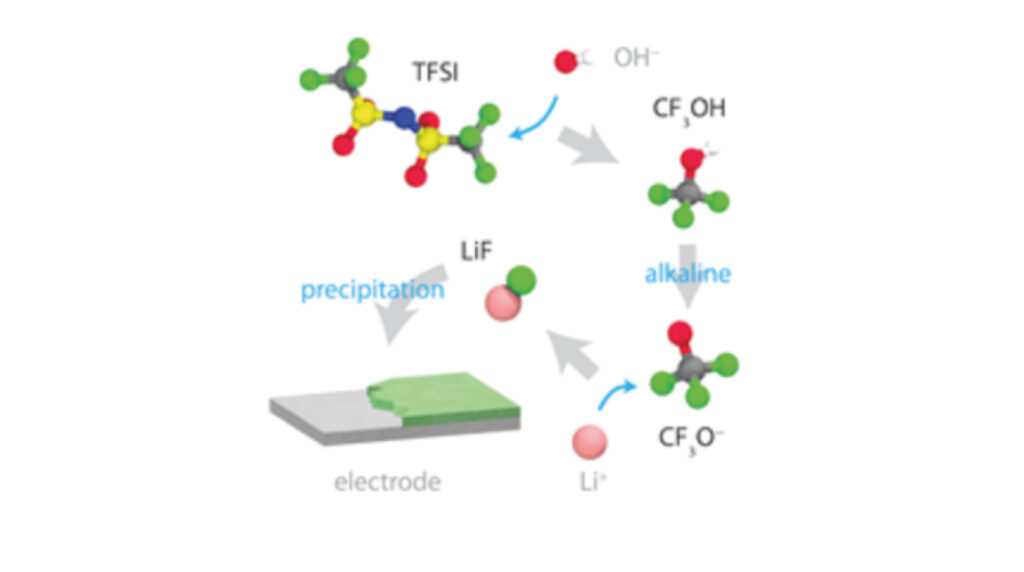
Water-in-salt electrolytes are attractive for high-voltage aqueous batteries since a LiF-based solid electrolyte interphase provides protection against electrolyte degradation during cycling and storage. The mechanism of formation for the solid electrolyte interphase in basic conditions is not fully understood. Here, the authors use molecular dynamics simulations to test a formation mechanism hypothesis, derived from experiment, in which the bistriflimide (TFSI) anion reacts with hydroxide anions that are produced in situ to form LiF. Surprisingly, the results show the formation of a reaction intermediate, trifluoromethanol, a well-known fluorinating agent, instead of fluoroform, which was previously expected but not detected. Gaining control over the formation of the reaction intermediate can impact design and analysis of electrolytes for aqueous batteries.