MER
Electroanalytical Methodologies and Reactivities
Objective: Study of charge storage mechanisms and reactivities at interfaces in aqueous media.
Research areas:
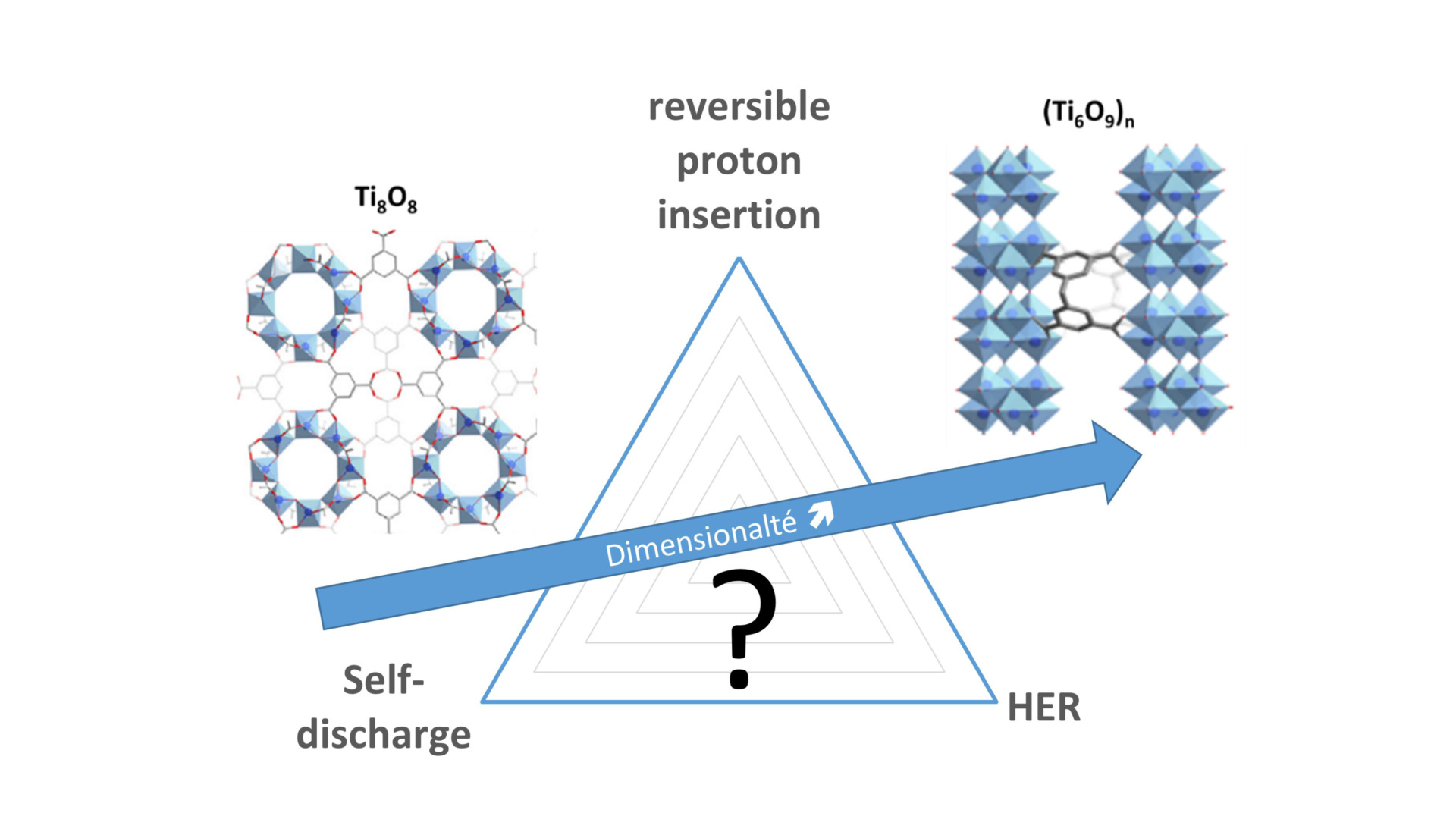
- Investigating Proton-coupled electron transfer reactions at various redox-active materials: from metal oxides (TiO2, MnO2, WO3…) to organic composites/polymers;
- Analysis of proton specificity as a charge carrier, mono/multivalent cation-proton competition;
- Metal electrodeposition/electrodissolution processes in aqueous electrolytes;
- Development of electroanalytical methodologies and multiphysics modelling tools; – Bifunctional electrochromic devices (smart windows).
Members
- Balland Véronique
- Limoges Benoît
- Branca Mathieu
- Sangarane Renald
- Wenkang Wang
Publications
- Impact of Reversible Proton Insertion on the Electrochemistry of Electrode Materials Operating in Mild Aqueous Electrolytes: A Case Study with TiO2. N. Makivic, K. D. Harris; J-M. Tarascon, B. Limoges, V. Balland (2022) Adv. Energy Mater., 2203122.
- Identifying interfacial mechanisms limitations within aqueous Zn-MnO2 batteries and means to cure them with additives. Y. Aguilar, P. Lemaire, N. Ayouni, E. Bendadesse, A. V. Morozov, O. Sel, V. Balland,* B. Limoges, A. M. Abakumov, E. Raymundo-Piñero, A. Slodczyk, A. Carnizares, D. Larcher, J-M. Tarascon (2022) Energ. Stor. Mater. 53, 238-253.
- Evidence of Bulk Proton Insertion in Nanostructured Anatase and Amorphous TiO2 Electrodes. N. Makivic, J-Y Cho, K. D. Harris; J-M. Tarascon, B. Limoges, V. Balland (2021) Chem. Mater. 33, 3436-3448
- The role of Al3+-based aqueous electrolytes in the charge storage mechanism of MnOx cathodes. V. Balland, M. Mateo, A. Singh, C. Laberty-Robert, K. D. Harris, B. Limoges (2021) Small 17, 2101515.
- Accessing the Two-Electron Charge Storage Capacity of MnO2 in Mild Aqueous Electrolytes. M. Mateos, N. Makivic, Y-S Kim, B. Limoges, V. Balland (2020) Adv. Energy Mater. 10, 2000332.
- Evidencing Fast, Massive and Reversible H+ insertion in Nanostructured TiO2 Electrodes at neutral pH. Where Do Protons Come From ? Y-S Kim, S. Kriegel, K.D. Harris, C. Costentin, B. Limoges, V. Balland (2017) J. Phys. Chem. C 121, 10325-35.